A new study reveals a novel function for the cytosolic protein Cypin in regulating synaptic composition and function via K63-linked polyubiquitination—an underexplored post-translational modification in neurons. The work answers key questions about how specific ubiquitin chains modulate synaptic targeting and turnover, offering insights with therapeutic potential.
Background: Ubiquitination as a Synaptic Regulator
Synaptic function relies on tightly controlled protein turnover, especially during learning and memory. Ubiquitination, a post-translational tagging system, orchestrates this by labeling proteins for degradation or modulating their trafficking and signaling roles. While K48-linked polyubiquitination is classically associated with proteasomal degradation, K63-linked chains are non-degradative and mediate signaling, trafficking, and autophagy.
In neurons, K63-polyUb has been linked to increased scaffolding of PSD-95 and AMPA receptor regulation, yet its mechanisms of regulation have remained elusive—until now.
Cypin Interferes with the Proteasome and Skews Ubiquitination
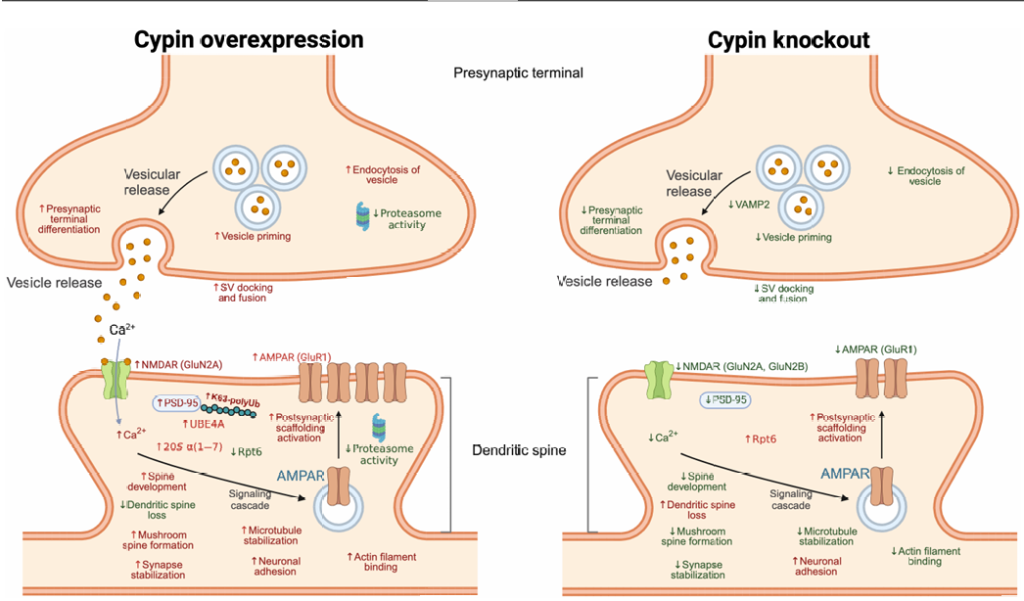
The study identifies Cypin (cytosolic PSD-95 interactor) as a key modulator of the proteasome and K63-polyubiquitination:
Interacts with PSMB4, a β7 subunit of the proteasome.
Inhibits proteasomal activity, reducing chymotrypsin-, trypsin-, and caspase-like activities.
Reduces 20S and 19S proteasome subunits (e.g., Rpt6) in vitro and in vivo.
Promotes K63-polyUb while suppressing K48-polyUb in developing neurons and at synapses.
Mechanistically, a proteomic screen shows that Cypin upregulates UBE4A, an E4 ubiquitination factor known to promote K63 linkages—implicating it as a mediator of this effect.
Cypin Interferes with the Proteasome and Skews Ubiquitination
The study identifies Cypin (cytosolic PSD-95 interactor) as a key modulator of the proteasome and K63-polyubiquitination:
Interacts with PSMB4, a β7 subunit of the proteasome.
Inhibits proteasomal activity, reducing chymotrypsin-, trypsin-, and caspase-like activities.
Reduces 20S and 19S proteasome subunits (e.g., Rpt6) in vitro and in vivo.
Promotes K63-polyUb while suppressing K48-polyUb in developing neurons and at synapses.
Mechanistically, a proteomic screen shows that Cypin upregulates UBE4A, an E4 ubiquitination factor known to promote K63 linkages—implicating it as a mediator of this effect.
Implications for Development, Injury, and Therapy
Neurodevelopment: Cypin is involved in dendrite and spine formation and is upregulated following neuronal activity.
Trauma: Elevated after traumatic brain injury (TBI); Cypin activation can restore memory performance in TBI models.
Plasticity: Overexpression doubles presynaptic activity—supporting its role in circuit rewiring.
Therapeutic Outlook: Modulating Cypin levels or downstream ubiquitin machinery may be a viable approach for restoring synaptic plasticity in neurological disorders.
Future Directions
This work opens several lines of investigation:
Dissecting UBE4A’s role in Cypin-mediated K63-polyUb.
Exploring behavioral consequences of altered synaptic K63-polyUb signaling.
Clarifying interplay between degradative and non-degradative ubiquitin linkages in neural circuits.
Conclusion
This study uncovers Cypin as a master regulator of synaptic proteostasis via selective K63-linked polyubiquitination and proteasome modulation. The findings extend our understanding of post-translational control at synapses and open new avenues for therapeutic targeting in neurodevelopmental and neurodegenerative conditions.
You can read the complete article here.